
Management of Radiation-Induced Vesicovaginal Fistula
Article authors



Department of Urology; Moscow State Medical Stomatological University;
127206, 21/3, Vucheticha, Moscoiu, Russia
Abstract
Background: Pelvic radiation used for the treatment of malignant diseases is the primary cause of delayed vesicovaginal fistula.
Objective: We consider the Martius labial fatty flap technique and the Latzko upper colpocleisis as important tools for the urologist dealing with patients suffering from radiation-induced vesicovaginal fistula (RWF).
Design, setting, and participants: In our clinic, 216 patients with RWF underwent surgical treatment. The vaginal approach was used in 210 cases (97.2%), and the abdominal approach was used in 6 cases (2.8%). The Martius flap technique was used in 41.0% of cases (86/210). In 35.7% of cases (75/210), the fistula repair was done by Latzko colpocleisis.
Surgical procedures: Martius procedure includes mobilization of labial flap and its interposition between bladder and vaginal walls during fistula repair. In Latzko upper colpocleisis, only vaginal mucosa around the fistula is excised. Then fistula is closed with several layers through anterior and posterior vaginal walls obliterating the upper vagina.
Measurements: Primary RWF repair was effective in 48.1% patients (101/ 210). One hundred and nine patients (51.9%) had failed surgery and remained incontinent. Ninety-eight patients were operated on again with cumulative success rate reaching 66.6% (140/210). Forty-two patients underwent tertiary surgery and 19 patients had more than three operations.
Results and limitations: Taken together, 169 patients out of 210 have had their RWF successfully closed. This resulted in an overall efficiency of 80.4%. Presented data include our experience of more than 40 yr that limits standardization of treatment and lack of some data.
Conclusions: The closure of the fistula may be done in several steps by reducing the size of the fistula and giving patients more time to recover. The Martius labial flap is a safe and effective procedure for these cases. Latzko upper colpocleisis is preferable when the risk of ureteral damage during surgery is present.
1. Introduction
Vesicovaginal fistulae remain one of the most challenging problems in modem female urology. Although it is not a life-threatening problem, vesicovaginal fistulae nonetheless significantly decreases a woman’s quality of life. Fortunately, it has become a rare problem in western countries due to improvements in obstetric standards. At the same time, radiation-induced vesicovaginal fistulae (RWF) have recently become more apparent, despite the fact that radiation therapy on pelvic organs is now less aggressive and more precise.
The modern use of ionizing radiation in gynecology is limited to the treatment of malignant diseases. Pelvic radiation is the primary cause of delayed vesicovaginal fistula [1]. When radiation is terminated, fibrosis occurs in the bladder lamina propria. Due to these changes, hyalinization of the connective tissues develops. Histologic examinations show the presence of large bizarre fibroblasts, which are described as radiation fibroblasts [2]. Small and medium arteries are affected by radiation-induced obliterative arteritis. Vascular damage of bladder tissue leads to atrophy or necrosis of the bladder epithelium, which causes ulceration, or the formation of fissures.
The majority of fistulae become apparent 1.5-2 yr after termination of radiotherapy. Some fistulas may not appear for many years after treatment [3]. The presence of a typical RWF is preceded by radiation cystitis, fever, and hematuria. These symptoms change dramatically with the sudden presence of vesicovaginal fistula. The tissues surrounding the fistula are indurated and bleed easily. Ulcerations of mucosa and areas of necrosis on the bladder and vaginal walls are the typical finding during clinical evaluations of RWF patients. It is obvious that the success of any plastic surgery is extremely limited in this situation due to lack of tissues.
2. Patients and methods
2.1. Patients
Since 1962, the Urology Department of Moscow State Medical Stomatological University has served as a dedicated centre for female urology in the Russian Federation. During this time, 216 patients suffering RWF have had surgical repairs performed in our clinic. The mean age of the patients was 57.2 yr (range 23-81 yr, standard deviation [SD] 13.4). A hysterectomy, followed by external beam radiation, was initiated in these patients because of cervical carcinoma.
Due to different standards of radiation therapy implemented during the last 40 yr, it is almost impossible to divide these
patients into groups according to treatment regiments. Generally, 86.1% of the females (186 patients) got a cumulative dose of radiation from 40-60 gray. Another 26 women (12.0%) had a total dosage of radiation less than 40 gray. The radiation regiment of four patients was unclear (1.85%). The period of time from the last radiation therapy to PWF development varied between 3 mo and 10 yr, and averaged to 21.2 mo (SD 24.3).
First of all, the surgeon must prove that there is no local recurrence of malignant disease in fistula edges. Multiple focal biopsies are obligatory in this stage. Upper urinary tract functions should be carefully evaluated. Intravenous pyelography helps to rule out an involvement of ureters and kidney damage. Cystoscopy is a crucial method to demonstrate the location and size of the fistula as well as proximity to ureteral orifices. Cystoscopy also helps to assess the bladder mucosa for edema and persistent necrosis, which may complicate planned surgical repair. The presence of radiation-induced uretero-vaginal fistula is a separate matter of dispute, outside the frame of this article. In most cases, ureteral injuries due to radiation are limited to sclerosis of paraureteral tissues and obliteration of lower ureters or ureteral orifices. In some cases, ureteral orifices appear on the edges of vesicovaginal fistula.
In our group of patients, an average period of primary RWF repair was 14.8 mo (from 11-18 mo after fistula formation). Standard preoperative evaluations of these patients included physical examination, urethrocystoscopy, intravenous pyelo-gram/ultrasound, and urinalysis.The transvaginal route was preferred for repair wherever possible, but the transabdominal route was adopted for repair of RWF in patients with significant scarring and shrinkage of vagina due to radiation. The size of the fistulae ranged between 1-2.5 cm in the vast majority of the patients (192/216). The remaining 24 patients developed larger fistulae with more extensive damage of vaginal and bladder walls. Primary fistula repair by vaginal approach was used in 210 cases (97.2%). Only in six cases (2.8%) was the abdominal approach with omentum flap performed. Bladder capacity and compliance were investigated prior to surgery during cystoscopy. We used a vaginal Foley catheter to occlude the fistula during cystoscopy in order to avoid fluid leakage and fill the bladder properly. Thirty-two patients (14.8%) had decreased bladder capacity (<100 ml). Only two of the patients required bladder augmentation due to complete loss of bladder capacity (0.93%).
In 35.7% of cases (75/210 patients) the fistula repair was amplified by Latzko colpocleisis. The Martius flap interposition is one of the key points for successful repair of RWF. It was used in 86 (41.0%) patients (out of 210) when the vaginal approach was chosen for fistula management. We present this technique in the DVD enclosed with this article.
2.2. Surgical technique
The surgery starts with patient in the dorsal lithotomic position. The Trendelenburg position can improve visualization of the vesicovaginal fistula. A16 or 18 F Foley catheter is placed into the bladder. Vaginal examination aims to eliminate the risk of missing any additional small fistulas not found during routine vaginal exams without anesthesia, and to evaluate the condition of surrounding tissues. As a result of severe pain, proper vaginal examination with a vaginal speculum is possible only under anesthesia. This fact shifts decision-making about the volume of surgical interventions and prediction of outcomes to the perioperative period as far as vaginal exams done under anesthesia is concerned. Therefore, it is extremely important to discuss with the patient possible treatment options and consequences prior to surgery.
The principles of vesicovaginal fistula surgical repair can be summarized in three points: (1) excision of all scar tissues; (2) splitting of vaginal and bladder layers; and (3) the closure of the fistula without the overlapping of the suture lines. These principles are not fully applicable for radiation-induced fistulae management: The area of vaginal and bladder walls surrounding the fistula is always scarred due to radiation and excision of the fibrotic tissues, and would result in large defects on the area of fistula. When this is the case, Latzko colpocleisis should be applied. Another justification for the Latzko technique is the risk of damaging ureters during preparation of the scarred bladder wall during manipulations on the bladder wall in the first stage of fistula repair.
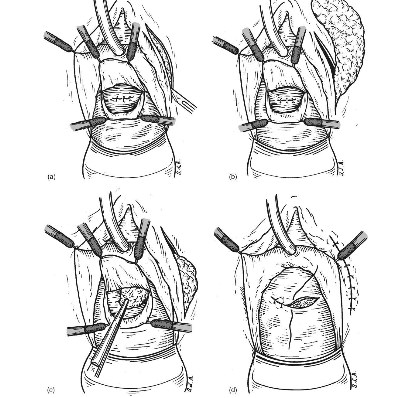
Fig. 1 - (a) Martius flap technique: A longitudinal indsion is made on labia majora pudenda, (b) Martius flap technique: Vascularized labial fatty flap is mobilized, (c) Martius flap technique: The flap is tunneled under the vaginal wall from labia to vaginal indsion. (d) Martius flap technique: The vaginal wall is dosed over the flap.
2.2.1. Fistula repair
A circumferential incision is made around the fistula to separate and mobilize bladder and vaginal walls. Extensive excision of the fistula tissue in these patients should be avoided, as this may lead to lack of tissue. We do not advise a catheterization of ureters prior or during surgery as a routine procedure. However, whenever a large mobilization of the bladder wall is required for repair of fistula, it might be safe to insert ureteral catheters during operations and remove them as soon as possible afterwards. In some cases, when ureteral orifices are localized on the edges of fistula, and there is a risk of ureteral obstruction through postoperative edema of the surrounding tissues, JJ ureteral stents are applicable. Once the bladder wall is mobilized, sutures are placed on it, preferably in a transverse fashion. Once the first line of sutures is completed, evaluation of the bladder wall with a metallic female catheter allows the surgeon to see the small defects, if any, in the suture line, which must be closed properly in a watertight manner. A second suture line is placed using perivesical tissue. The second layer must cover the first layer as completely as possible. Indeed, this is not always possible due to surrounding fibrotic changes. Interpositional tissue should be considered whenever the closure lines or vaginal tissues are of questionable quality.
2.2.2. Martius flap technique
The surgical technique of labial flap harvesting was described by Martius in 1928 [4]. The procedure starts with a lateral incision on the labia majora pudenda (Fig. la). The side from which the flap is taken depends on fistula location. When choosing the side, the shortest distance to the covering area has to be considered because of less tension, a better blood supply for the flap, and better recovery with less recurrence. When a longitudinal incision on the labia majora is done, a well vascularized labial fat is exposed (Fig. lb). The mobilization of the graft should be done taking into consideration the pudendal or epigastric blood supply. From its ventral aspect, the fat graft is supplied by epigastric vessels, and, if the base of the flap is dorsal, the circulation is furnished by the pudendal basin [5]. These features make the Martius flap a flexible tool for fistula management. After mobilization, the flap is tunneled by a curve clamp under the vaginal wall, from labia to vaginal incision (Fig. lc). We use absorbable sutures for fixing the flap over the fistula repair site. Normally the labial flap has excellent mobility and significant bulk effect. This bulk effect is often quite necessary, but should be considered before flap harvesting. Excessive bulking may provoke problems with the anterior vaginal wall closure. This is why the vaginal wall should be mobilized enough in order to cover the flap without tension. The incision over the labia majora pudenda is sutured (Fig. Id). The final step of the procedure is vaginal wall closure. Any absorbable 3.0 synthetic suture may be used. A Foley catheter stays in place for an average 2 wk. All patients receive broad spectrum antibiotics for 1 wk. They may be discharged from the hospital on the second day post-operatively.
2.2.3. Latzfeo colpocleisis
The Laztko technique, described in 1942 [6], is the treatment of choice for the patient suffering from small radiation-induced vesicovaginal fistula with significant trophic disturbances of vaginal and bladder wall, which do not allow the surgeon to perform traditional splitting repair. The excision of the vaginal epithelium around the fistula site in an oval manner is the first step of the surgery (Fig. 2a). It should be empathized that only vaginal mucosa is excised without going deeper to bladder wall. This maneuver allows the prevention of any ureteral or bladder trigone injuries during operations. In some cases, additional incisions on the lateral parts of oval wound are needed for making reattached surfaces of the vaginal wall larger. The second stage of the procedure includes a closure of fistula with several layers of absorbable sutures (Vicril 3.0). The sutures pass through anterior and posterior vaginal walls, obliterating upper vagina (Fig. 2b and 2c). Due to the fact that the bladder is not involved in the surgery, ureteral reimplantation should not be required, even if ureteral orifices are localized on the edges of fistula while no damage of upper urinary tract is present. The Latzko procedure may be classified as upper colpocleisis; therefore, the vaginal depth and sexual functions are not significantly compromised by it [3]. In the case of a large fistula, or if there has been significant injury to adjacent areas, additional tissue flaps may be necessary to achieve a proper repair.
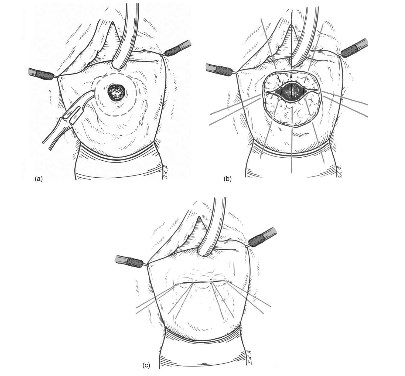
Fig. 2 - (a) Latzko procedure: Excision of the vaginal epithelium around the fistula is made, (b) Latzko procedure: Sutures pass through anterior and posterior vaginal walls, (c) Latzko procedure: Fistula ia closed by upper colpodeisis.
3. Results
We analyzed the outcome results of 210 patients who underwent fistula repair by vaginal approach. The overall success rate after primary RWF repair was 48.1%, or 101 continent patients. One hundred and nine patients (51.9%) had failed surgery and remained incontinent. Eleven patients (10.01%) among those 109, for whom the primary management did not succeed, refused further attempts to close fistula or were referred to other centres.
Ninety-eight patients accepted secondary surgery, and were operated on within an average period of 6 mo after previous operations. Success was achieved in 39.8% (39/98) and the cumulative rate was 66.6% after secondary surgery (140/210). Most patients (42/59; 71.2%) on whom secondary attempts to close the fistula were not successful were operated on again. Seventeen females refused additional surgical treatment and the dropout rate after second operations on RWF reached 28.8%. This number is significant, as it means that every fourth patient who remains incontinent after second attempts to close fistula refuses more surgery and accepts her current status. We have to empathize here that the size of fistula for most of these patients reduced significantly after repair, resulting in better quality of life even if there was recurrence.
Forty-two patients underwent tertiary surgery with a closure rate of 52.4% (22 cases). By this stage, 162 patients out of 210 were treated, with a cumulative success rate of 77.1%. Only 19 patients had more than three operations to manage radiation-induced vesicovaginal fistulae. In these patients, successful closures were achieved in seven cases (35.0%). Taken together, 169 patients out of 210 had their RWF successfully closed. This resulted in overall 80.4% efficiency.
4. Discussion and conclusions
Pelvic radiation for treatment of cervical carcinoma is the primary cause of radiation-induced vesicovaginal fistula. Maier et al [7] reported that 1.24% (133/ 10 709) of the patients who underwent radiation therapy suffered severe urologic complications such as vesicovaginal fistula that required surgical intervention. Considering the high prevalence of cervical carcinoma and wide use of radiation therapy, the management the vesicovaginal fistula becomes an important issue for modem female urology.
Since every fistula is unique and requires an individualized approach, it is difficult to describe a standard fistula repair, but some useful tips and general principles can be summarized. First, surgery should be avoided until an acute postradiation tissue response is progressing. A fistula occurring in a radiated field should be periodically reassessed, given the propensity of these lesions to evolve over time. In our experience, the minimal timing prior to repair approximates 12 mo since the fistula first appeared. Lack of tissue which can be mobilized is the main problem the surgeon faces during radiation-induced fistula repair. If this is the case, one should use flap techniques in order to create a "waterproof’ closure [8]. Martius labial fatty flap is a convenient and safe procedure for these patients. Latzko upper colpocleisis is preferable when the risk of ureteral damage during surgery is present due to significant scarring of vaginal and bladder walls and localization of the fistula close to ureteral orifices. Proper informed consent from the patients should be obtained, taking into consideration high risk of fistula recurrence, sexual dysfunctions, or obstructive ureteral complications. In our experience, the closure of the fistula may be done in several steps by reducing the size of the fistula, and giving the patient more time to recover well. On average, only half the patients are cured after one surgical procedure. Subsequent repairs do not decrease the patients’ chances to be cured and a cumulative rate of cure is still high. It is necessary to emphasize that failure of fistula repair is mostly due to continuing tissue reaction caused by radiation. Because of this particular point each next fistula repair in case of failure may be considered as “primary” surgery. There is no specific difference between surgical techniques of primary or subsequent fistula management.
Urologic complications are major side-effect of radiation therapy for cervical cancer. Eifel et al [9] showed that there is 9.3% probability of having a major urinary tract or gastrointestinal complication after cervix radiation therapy. There was a subsequent continuous risk of approximately 0.34% per year, resulting in risk of major complications of 11.1% at 10 yr, 13% at 15 yr, and 14.4% at 20 yr. Authors suggest that continued surveillance of patients is important, because radiation tissue injury could progress over time. Drutz suggested that minimum of 6 mo is required prior to surgery for postirradiation fistulae [10]. Our experience shows that initial stabilization of radiation reaction happens within a year after the therapy. So in some cases delayed repair is advisable [11,12]. And it remains our opinion that the majority of RWF can be repaired successfully via the vaginal route (Table 1).
Table 1 - Outcomes of surgical managements of radiation-induced fistulae
| Primary surgery (210 pts.) | Secondary surgery (98 pts.) | Tertiary surgery (42 pts.) | More than 3 surgeries (13 pts.) | |
| Successful closure of fistula | 101 | 39 | 22 | 7 |
| Failure | 109 | 59 | 20 | 6 |
| Refused surgery | 0 | 11 | 17 | 7 |
| Success rate on each stage | 48.1% | 39.8% | 52.4% | 53.8% |
| Cumulative success rate | 48.1% | 66.6% | 77.1% | 80.4% |
Radiation-induced vesicovaginal fistulae are one of the most challenging problems in modem female urology. Martius labial flap and Latzko upper colpocleisis techniques are essential for urologists dealing with patients suffering from this problem.
Management of radiation-induced vesicovaginal fistulae is a complex issue, which demands time and major efforts from both medical personnel and patients.
Author contributions: Dmitri Y. Pushkar and Vladimir V. Dya-kov had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pushkar, Kasyan.
Acquisition of data: Pushkar, Dyakov.
Analysis and interpretation of data: Pushkar, Kasyan.
Drafting of the manuscript: Pushkar, Kasyan.
Critical reuision of the manuscriptfor important intellectual content: Pushkar, Dyakov, Kasyan.
Statistical analysis: Pushkar, Kasyan.
Obtaining funding: None.
Administrative, technical, or material support: Pushkar, Dyakov, Kasyan.
Supervision: Pushkar.
Other (specify): None.
Financial disclosures: None.
Funding/Support and role of the sponsor: None.
References
- Graham JB. Vaginal fistulas following radiotherapy. Surg Gynecol Obstet 1965;120:1019-30.
- Berthrong M. Pathologic changes secondary to radiation. World J Surg 1986;10:155-70.
- Raz S, Little NA, Juma S. Female urology. In: Walsh PC, Retik AB, Stamey ТА, editors. Campbell’s Urology. 6th ed. Philadelphia: WB Saunders; 1992. p. 2782-828.
- Martius H. Die operative Wiederherstellung der volkom-men fehlenden Harnrehre und des Schiessmuskels der-selben. Zentralbl Gynakol 1928;52:480-6.
- Hoskins WJ, Park RC, Long R, et al. Repair of urinary fistulas with bulbocavernosus myocutaneous flaps. Obstet Gynecol 1984;63:588-91.
- Latzko W. Postoperative vesicovaginal fistulas: genesis and therapy. Am J Surg 1942;58:211-28.
- Chappie C, Turner Warwick R. Vesicovaginal fistula. Br J Urol 2005;95:193-214.
- Maier U, Ehrenbock PM, Hofbauer J. Late urological complications and malignancies after curative radiotherapy for gynecological carcinomas: a retrospective analysis of 10,709 patients. J Urol 1997;158:814r-7.
- Eilber K, Kavaler E, Rodriguez L, Rosenblum N, Raz S. Ten-year experience with transvaginal vesicovaginal fistula repair using tissue interposition. J Urol 2003;169:1033-6.
- Eifel PJ, Levenback C, Wharton JT, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1995;32:1289-300.
- Drutz HP, Herschom S, Diamant NE. Female Pelvic Medicine and Reconstructive Surgery. New York: Springer; 2003.
- Blaivas JG, Heritz DM, Romanzi LI. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol 1995;153:1110-3. Editorial Comment on: Management of Radiation-Induced Vesico-vaginal Fistula






