
Sculpturing in Urology, or How to Make Percutaneous Nephrolithotomy Easier
Article authors





1Department of Urology, VCERM, Saint Petersburg, Russian Federation.
2Department of Urology, Saint Elizabeth Hospital, Saint Petersburg, Russian Federation.
Abstract
Purpose: To investigate the usefulness of Plasticine biomodeling in surgical percutaneous management of complex renal stone.
Patients and Methods: A total of 32 patients with complex renal stones (complete staghorn stones or partial staghorn stone with multiple caliceal stones) were included in this study from 2012 to 2013. Computed tomography (CT) urography with three-dimensional (3D) reconstructions was used as standard preoperative imaging in all patients. Preoperatively, Plasticine replication of the pelvicaliceal system was performed by the operating surgeon, based on the gathered 3D reconstructions. Then the model was taken to the operating room and used as a reference model in a sterile polyethylene bag during the operation.
Results: Percutaneous renal access was achieved successfully in all cases. Twenty-nine (91%) patients were treated in the prone position and only 3 (9%) in supine position. There were 18 (56%) patients who had a single tract, 9 (28%) patients had two tracts, 3 (9%) patients had three tracts, and one (3%) patient needed four tracts. The mean operative time was 92 ( – 26) minutes. Second-look percutaneous nephrolithotomy (PCNL) was needed in 9 of 32 (28%) patients. All second-look sessions were performed in 2 to 3 days and/or on a normalized temperature. Six of 11 (54.5%) patients with complete staghorn stones needed a second-look PCNL session. Complete stone clearance was confirmed by low-dose CT, performed at 24 hours after surgery, in 89.4% of the patients treated by a single PCNL session and 82% in those who needed second-look sessions. The overall stone-free rate (SFR) in the study after second looks was 87.3%.
Conclusions: The proposed Plasticine 3D model seems to provide better preoperative renal collecting system appreciation and to serve as a reference tool during the operation, which in turn might increase SFRs and lower the complications rate after PCNL.
Introduction
Since the first case of percutaneous nephroscopy was presented by Rupel and Brown1 in 1941, percutaneous surgery went through significant improvements until today when it is the first-line surgical management for renal stones more than 2 cm.2
Adequate preoperative planning is mandatory for successful percutaneous nephrolithotomy (PCNL), which is based on consideration of the patient’s performance status, comorbidities, and optimal imaging. Today, preoperative imaging for the best depiction of renal calculi is computed tomography (CT) with or without contrast enhancement.3 A contrast study is recommended, however, if stone removal is planned and the anatomy of the renal collecting system needs to be assessed; moreover, three dimensional (3D) CT reconstruction of the collecting system, as well as measurement of stone density and skin-to-stone distance is possible.2
In that way, a better awareness of 3D anatomy of the pelvicaliceal system (PCS) with different CT reconstructing techniques is obtained.4
In the operating theater, preoperative CT scans, with 3D reconstruction of PCS and stone, on a monitor have several limitations: To keep confident orientation in the kidney during the operation one has to constantly keep in mind those 3D images of the renal collecting system or periodically be distracted to compare intraoperative findings with CT films.
To overcome this obstacle, several attempts were made by creations of a stereolithographic biomodel, using laser and 3D printing of the PCS.5,6 Despite the approved benefit, however, these techniques did not gain widespread recognition because their creation was time-consuming and necessitated additional expenditures.
Attempts to get a more efficient intraoperative reference tool have led to a simple idea of Plasticine biomodeling, which is created by the surgeon from a 3D CT reconstruction of the PCS. It is cheaper, faster to get, and, crucially, since it is made by the surgeon will give him or her a thorough and deep knowledge of the collecting system anatomy.
The aim of this study was to investigate the usefulness of Plasticine biomodeling in surgical management of complex renal stones.
Patients and Methods
After institutional ethical committee approval, a total of 32 patients with complex renal stones (complete staghorn stones or partial staghorn stone with multiple caliceal stones) were included in this study from 2012 to 2013. Patients with coagulation disorders and/or active infection were excluded from the study. Patient characteristics are shown in Table 1.
Preoperative laboratory tests included complete blood cell count, serum chemistry panel, coagulation screening tests, urinalysis, and urine culture. All patients presented either with sterile urine or were treated according to the antibiotic sensitivity tests for at least 7 days before the operation.7
CT urography with 3D reconstructions was used as a standard preoperative imaging in all patients. Data acquisition was performed using a 64-row CT unit Somatom Definition AS (Siemens, Germany) with the patient supine.
Stone types were classified as staghorn calculi, either with or without multiple caliceal stones. Complete staghorn stones were defined as stones occupying the renal pelvis and all the caliceal system or more than 80% of the renal collecting system. Whereas partial staghorn stones were defined as stones occupying the renal pelvis and at least two calices.8
Preoperatively, Plasticine replication of the PCS was performed by the operating surgeon, based on the gathered 3D reconstructions (Fig. 1). The average time needed for model creation was 20 – 6 minutes. The cost of Plasticine needed for a single model in Russia is less than $5, which is quite cheap. Stepwise depiction of the modeling process is shown in Figure 2. Then the model was taken to the operating room and used as a reference model in a sterile polyethylene bag during the operation (Fig. 3). The surgeons did not have any special training in sculpturing beforehand.
The same surgical team performed all PCNLs. With the patient under general anesthesia in the lithotomy position, a ureteral catheter was placed into the ipsilateral kidney under cystoscopic guidance. Subsequently, the patient was turned to the prone position, which is the preferred in our center, or left in the supine position in the case of morbid obesity or cardiac diseases. This was up to the discretion of the attending surgeon and anesthesiologist.
Percutaneous access was achieved under fluoroscopic guidance. After calix puncture, a 0.035 hydrophilic guidewire was inserted into the PCS. Maximum effort was made to direct the guidewire down the ureter, thus to have a through and through access. Predilation of the percutaneous tract was performed using an 11F fascial dilator, and the 8F radiopaque tetrafluoroethylene catheter was inserted over the guidewire. After a ‘‘single-step’’ tract dilation, a 30F sheath was introduced.
Nephroscopy was performed with a rigid 28F nephroscope (Karl Storz, Germany). Fragmentation of the stone burden was accomplished with an ultrasonic lithotriptor predominantly (Swiss LithoClast Master, Switzerland), which has a pneumatic lithotripsy option as well. A biprong forceps (Karl Storz, Germany) and/or PERC NCircle nitinol tipless stone extractor (Cook Medical, Bloomington, IN) were used to remove stone fragments when needed. Additional tracts were created during the same session if indicated. A total duration of nephroscopy was no more than 2 hours.
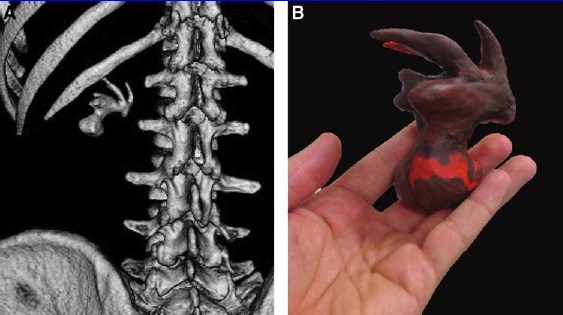
FIG. 1. Preoperative 3D reconstruction (A) and Plasticine biomodel (B) of the complete staghorn stone.
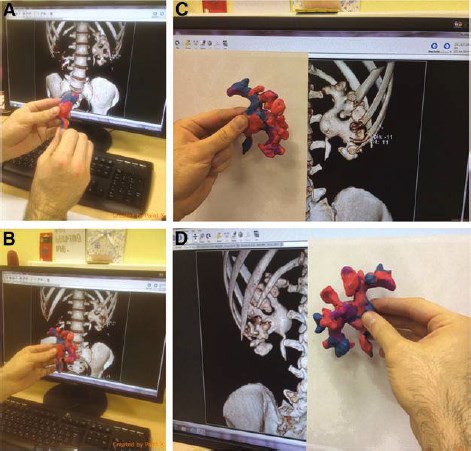
FIG. 2. Stepwise depiction of modeling. (A) Replication of the pelvis, (B) replication of posteriorly oriented calyces, (C) replication of anteriorly oriented calyces, and (D) checking the completeness and resemblance of the Plasticine model.
Table 1. Preoperative Patient Characteristics
Mean age, years54 ( – 7)
Sex
Male14 (44%)
Female18 (56%)
Stone type
Complete11 (34%)
Partial (with multiple caliceal)21 (66%)
Previous open renal stone surgeries9 (28%)
Laterality
Left12 (37.5%)
Right15 (46.8%)
bilateral5 (15.7%)
ASA class
18 (25%)
217 (53%)
37 (22%)
At the end of the procedure, the patient’s stone-free status was double-checked using fluoroscopy and a flexible nephroscope final inspection. A 9F nephrostomy tube was placed at the conclusion of most procedures. If intraoperative bleeding was a concern, then a large bore nephrostomy tube was considered. Peritubal tract infiltration with a local anesthetic, ropivacaine 0.25%, was used for postoperative pain alleviation.9 A determination of blood cell count, creatinine level, serum electrolytes, Foley catheter removal, and patient activation were performed on the first postoperative day
All patients were assessed for stone clearance at 24 hours after operation using a low-dose nonenhanced CT protocol. The stone-free status was defined as the absence of fragments on CT or clinically insignificant residual fragments, which are defined as less than < 4mm in diameter, in a noninfectious stone patient.
In patients who were considered stone free, the nephrostomy tube was removed 48 hours after operation after nephrostomy clamping or an antegrade contrast study proving free urine flow. The nephrostomy tube was left in place, however, if a second-look PCNL was planned because of the presence of residual stones. The operative time was defined as the time from the calix puncture to the final placement of a nephrostomy tube.
Evaluated parameters were stone-free rate (SFR) (with clinically insignificant residuals < 4 mm) and complication rates (modified Clavien grading system).10
Results
Percutaneous renal access was achieved successfully in all cases. There were 29 (91%) patients who were treated in the prone position and only 3 (9%) in the supine position. Eighteen (56%) patients had a single tract, 9 (28%) patients had two tracts, 3 (9%) patients had three tracts, and one (3%) patient needed four tracts. The mean operative time was 92 ( – 26) minutes. Second-look PCNL was needed in 9 of 32 (28%) patients. All second-look sessions were performed in 2 to 3 days and/or on a normalized temperature. There were 6 of 11 (54.5%) patients with complete staghorn stone patients who needed a second-look PCNL session, whereas only 3 of 21 (14.2%) patients with partial staghorn stones needed a second-look session.
Complete stone clearance was confirmed by low-dose CT, performed at 24 hours after surgery, in 89.4% of the patients treated by a single PCNL session and 82% in those who needed second-look sessions. The overall SFR in the study after second looks was 87.3% (Table 2).
Overall, 12 (37.5%) patients experienced postoperative complications. According to the Clavien classification: six patients (18.7%) presented grade I, four patients (12.5%) grade II, and two patients (6%) grade III complications. Fortunately, no serious complications occurred during the study. The grade I list was predominantly transient postoperative fever, which was found in five (15.7%) patients. In one (3%) patient, transient creatinine rise was detected. Grade II complications were hemorrhage in two (6.25%) patients, which necessitated blood transfusions after the procedure without any need for additional measures, such as super-selective angioembolization, etc. This complication was encountered in patients with complete staghorn stones after an operation of prolonged duration (although no operation lasted more than 2 hours) and with multitract access gaining. Postoperative fever ( > 38!C)in two (6.25%) patients was successfully managed with additional antibiotic administration.
Grade III complications occurred in one (3%) patient who underwent chest tube placement for pneumothorax and was from 11th intercostal access, and by another (3%) patient who needed stent placement for clot ureteral obstruction in the early postoperative period (Table 3).
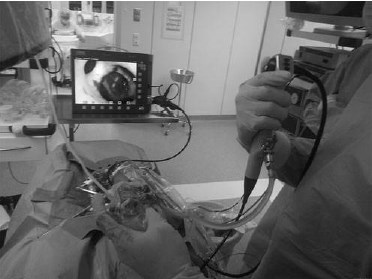
FIG. 3. Intraoperative navigation.
Table 2. Percutaneous Nephrolithotomy Characteristics
Mean operative time92 ( – 26) min.
Position
Prone29 pts (91%)
Supine3 pts (9%)
Second-look PCNL9 pts (28 %)
With complete staghorn stone6 of 11 (54.5%)
With partial staghorn stones3 of 21 (14.2%)
Tract number
118 pts (56%)
29 pts (28%)
33 pts (9%)
41 pt (3%)
Complete clearance by a single PCNL session89.4%
Complete clearance by more than one PCNL session82%
Overall stone clearance in the study87.3%
Table 3. Postoperative Complications (Clavien Classification)
Overall complications encountered12 pts (37.5%)
Grade I6 pts (18.7%)
Transient postoperative fever?5 pts (15.7%)
Transient creatinine rise1 pt (3 %)
Grade II4 pts (12.5%)
Hemorrhage2 pts (6.25%)
Postoperative fever > 38?2 pts (6.25%)
Grade III2 pts (6%)
Pneumothorax1 pt (3%)
Ureteral stent placement for clot ureteral obstruction1 pt (3%)
Discussion
According to existing guidelines, PCNL is the primary procedure for the treatment of patients with renal stones more than 2 cm. The success of PCNL largely depends on adequate planning, meticulous technique, and confident knowledge of the renal collecting system anatomy.6 Adequate planning, the mainstay, depends on optimal imaging, which are predominantly intravenous urography (IVU) or CT. Nevertheless, because CT provides superior detection capabilities for renal and ureteral stones, it has progressively replaced IVU in many institutions.11,12
Successful management of a renal calculus depends on a relationship of the calculus with individual pelvicaliceal anatomy and necessitates mental reconstruction of the PCS during percutaneous access and stone removal. This relationship can be gained by 3D CT reconstruction of the caliceal anatomy and stones, which undoubtedly helps to select the most appropriate approach.4 Thus, enhanced CT with volume rendering techniques is the preferred imagingmodality in our andmany other centers in complex stone cases. Rendered images provide a clear representation of the anterior and posterior direction of the calices, information that was impossible to determine using the IVU. The combination of the diagnostic accuracy of CT with 3D modeling for appreciation of the calculus location and caliceal anatomy may displace the IVU completely.13
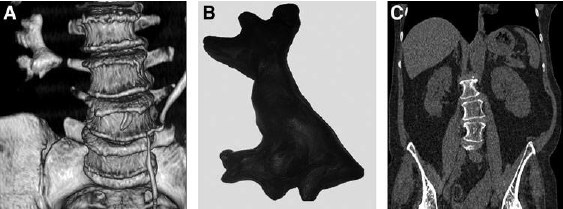
FIG. 4. Before and after perdutaneous nephrolithotomy: (A) three-dimensional reconstruction; (B) Plasticinemodel; (C) postoperative CT.
During PCNL, especially dealing with complex renal stone cases, the surgeon needs to keep in mind continuously CT volume reconstructions; that is not so easy, considering that human collecting systems can be very varied. Taking into account abovementioned access gaining and PCS navigation sometimes can be quite a challenging task.
The overall SFR of PCNL found in the Clinical Research Office of the Endourological Society PCNL database was only 76%.14 Given this percentage, ways for improvement are still needed. Recent reports trying to solve this problem such as an iPad-assisted navigation, 3D-augmented virtual reality,15 the locator system,16 Real-Time Tracking Sensors17 are reflecting the real need to gain intraoperative confidence, which is today is not ideal.
Another direction to improve SFR are attempts to create renal collecting system replication using different techniques: 3D printing, although this was used as a step in creation of a percutaneous access trainer,6 3D resin biomodel created by liquidbed laser curing system,5 and it has been suggested that a 3D biomodel may be helpful in accurate access puncture planning.5
Although recent advances in technology have enabled production of 3D printed models, their production still needs additional equipment such as a 3D printer and a necessity for special converting software, which imposes additional costs. The liquid-bed laser curing system used to produce the biomodel from plastic photopolymer resin also needs additional expenditures. Moreover, it is time-consuming, requiring about 2 to 3 days for biomodel production. These are main obstacles hindering its widespread use.
For some time, the idea of biomodeling has been used in different fields of medicine such as preoperative planning in complex orthopedic, craniofacial, and neurosurgical procedures. 18–20
The question of whether to combine biomodeling, which will alleviate the need for mental reconstruction of pelvicaliceal anatomy and improve collecting system anatomy recognition was raised. An interesting experience in the field of medical teaching has attracted our attention. Using a model of the hip joint, participants attached the structures out of Plasticine to the bony landmarks. In their experience, Plasticine models created an interactive learning experience that was relevant to surgical practice. Participants felt an improvement in their appreciation of 3D anatomical associations.21
We took the same principle and used a Plasticine PCS biomodel from 3D CT reconstructions before each operation was performed. Creation of a model took no more than 20 minutes on average. By creating the model, the surgeon acquires a perfect comprehension of the renal collecting system anatomy, sensation of a so-called intrarenal road map that is extremely important, especially in the management of complex renal stones. This model was taken into the operating room and placed in the sterile transparent polyethylene bag, which allowed its use intraoperatively as a reference tool if needed. 3D CT preoperative scans, with corresponding models and postoperative scans are shown in Figure 4.
Plasticine biomodeling according to our preliminary results and our sensation was very useful in the management of complex renal stones by PCNL by providing obvious benefit for planning and intraoperative technique when used as a reference tool during the operation. Thus, improvement in preoperative planning and operative technique might lead hypothetically to the shortening of operation duration, minimization of blood loss, risk of infection, and postoperative complications as well.
Some obvious drawbacks of this technique have to be highlighted. First, there is concern for the radiation dose received by the patients. CT in many centers, however, is the standard in preoperative imaging, thus imposing no requirement for additional visualizing methods. The second concern is Plasticine on the hands, which is simply removed with warm water.
Plasticine modeling is fast to produce, cheap, and gives better appreciation of the collecting system anatomy that might improve SFR, reducing operative time and complications.
Conclusion
The proposed Plasticine 3D model seems to provide better preoperative renal collecting system appreciation and serves as a reference tool during the operation, which in turn might increase SFR and the lower complications rate after PCNL.
Acknowledgments
We are very thankful to Arvind Ganpule, Vladimir Khvorov, Mohammed Lezrek, and John J. Knoedler for article revision and advice given.
Disclosure Statement
No competing financial interests exist.
References
1. Rupel E, Brown R. 1941. Nephroscopy with removal of stone following nephrostomy for obstructive calculous anuria. J Urol 1941;47:177–182. Available at: http://scholar .google.com/scholar?hl = en&btnG = Search&q = intitle: Nephroscopy + with + removal + of + stone + following + nephrostomy + for + obstructive + calculous + anuria#0. Accessed: August 13, 2014.
2. Tu¨rk C, Knoll T, Petrik A, et al. 2014. Guidelines on urolithiasis. European Association of Urology, pp.62–99. Available at: http://www.uroweb.org/gls/pdf/22%20Urolithiasis _LR.pdf. Accessed: October 30, 2014.
3. Olcott EW, Sommer FG, Napel S. Accuracy of detection and measurement of renal calculi: In vitro comparison of threedimensional spiral CT, radiography, and nephrotomography. Radiology 1997;204:19–25.
4. Ghani KR, Pitsher J, Patel U, Anson K. Three-dimensional imaging in urology. BJU Int 2004;94:769–773.
5. Radecka E, Brehmer M, Holmgren K, et al. 2006. Pelvicaliceal biomodeling as an aid to achieving optimal access in percutaneous nephrolithotripsy. J Endourol 2006;20:92–101.
6. Turney BW. A new model with an anatomically accurate human renal collecting system for training in fluoroscopyguided percutaneous nephrolithotomy access. J Endourol 2014;28:360–363.
7. Mariappan P, eSmith G, Moussa SA, Tolley DA. One week of ciprofloxacin before percutaneous nephrolithotomy significantly reduces upper tract infection and urosepsis: A prospective controlled study. BJU Int 2006;98:1075–1079.
8. Mishra S, Bhattu AS, Sabnis RB, Desai MR. Staghorn classification: Platform for morphometry assessment. Indian J Urol 2014;30:80–83.
9. Jonnavithula N, Pisapati MV, Durga P, et al. Efficacy of peritubal local anesthetic infiltration in alleviating postoperative pain in percutaneous nephrolithotomy. J Endourol 2009;23:857–860.
10. de la Rosette JJ, Opondo D, Daels FP, et al. Categorisation of complications and validation of the Clavien score for percutaneous nephrolithotomy. Eur Urol 2012;62:246–255.
11. Miller OF, Rineer SK, Reichard SR, et al. Prospective comparison of unenhanced spiral computed tomography and intravenous urogram in the evaluation of acute flank pain. Urology 1998;52:982–987.
12. Memarsadeghi M, Heinz-Peer G, Helbich TH, et al. Unenhanced multi-detector row CT in patients suspected of having urinary stone disease: Effect of section width on diagnosis. Radiology 2005;235:530–536.
13. Park S, Pearle MS. Imaging for percutaneous renal access and management of renal calculi. Urol Clin North Am 2006;33:353–364.
14. Kamphuis GM, Baard J, Westendarp M, de la Rosette JJ. Lessons learned from the CROES percutaneous nephrolithotomy global study. Available at: www.ncbi.nlm .nih.gov/pubmed/25100624. Accessed: August 14, 2014.
15. Rassweiler JJ, Mu¨ller M, Fangerau M, et al., iPad-assisted percutaneous access to the kidney using marker-based navigation: Initial clinical experience. Eur Urol 2012;61: 628–631.
16. Lazarus J, Williams J. The Locator: Novel percutaneous nephrolithotomy apparatus to aid collecting system puncture—A preliminary report. J Endourol 2011;25:747–750.
17. Rodrigues PL, Vilaca JL, Oliveira C, et al. Collecting system percutaneous access using real-time tracking sensors: First pig model in vivo experience. J Urol 2013;190:1932–1937.
18. Yau YY, Arvier JF, Barker TM. Technical note: Maxillofacial biomodelling—preliminary result. Br J Radiol 1995;68:519–523.
19. D’Urso PS, Askin G, Earwaker JS, et al. Spinal biomodeling. Spine 1999;24:1247–1251.
20. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med 2013; 41:894–902.
21. Asp A.R., Myint Y, Gandhe A. Back to school anatomy: Just add Plasticine. Available at: www.bmj.com/content/347/bmj.f6924. Accessed: August 14, 2014.






