
Tadalafil in the management of lower urinary tract symptoms: a review of the literature and current practices in Russia
Article authors




1Moscow State University of Medicine and Dentistry named after A.I. Evdokimov,
Department of Urology, Moscow, Russia
2Eli Lilly Vostok S.A.
3Eli Lilly and Company, Lilly Corporate Centre, USA
Introduction Strong epidemiologic evidence supports correlation between lower urinary tract symptoms due to benign prostatic hyperplasia (LUTS/BPH) and erectile dysfunction (ED). The link has biologic plausibility given phosphodiesterase type 5 (PDE5) expression in pelvic structures. PDE5 inhibitors target pathophysiologic processes implicated in LUTS/BPH.
Material and methods This review highlights the efficacy and safety of the daily use of a PDE5 inhibitor tadalafil in LUTS/BPH, with a focus on LUTS/BPH medical management in Russia.
Results Alpha–blockers and phytotherapy are major components of the current LUTS/BPH therapy in Russia. Russian regulatory authorities granted approval for once–daily tadalafil for treatment of LUTS/ BPH in January 2012. In a pivotal study, tadalafil 5 mg once–daily significantly improved International Prostate Symptom Score (IPSS) over 12 weeks vs. placebo (P = .004) regardless of baseline ED severity. IPSS improvement was maintained at 12 weeks. Integrated analysis of randomized studies showed that tadalafil 5 mg once–daily resulted in significant symptom improvements across a range of men with LUTS/BPH. Relief of LUTS due to tadalafil was independent of improvement in ED; improvements in IPSS and erectile function were only weakly correlated (r = –0.229). Another pooled analysis found similar improvement in LUTS/BPH between men with or without ED, with non–significant P values for treatment–by–ED–status interactions for total IPSS ( P = .73). Non–registration studies of tadalafil and alpha–blocker co–therapy in LUTS/BPH suggest an additive effect, but co–therapy is not recommended in current tadalafil prescribing instructions.
Conclusions Tadalafil results in symptom improvements across a range of men with LUTS/BPH and represents a new treatment option for patients in Russia with LUTS/BPH.
Key Words: tadalafil, PDE5 inhibitor, alpha–blocker, sexual function, Cialis, lower urinary tract symptoms/benign prostatic hyperplasia, erectile dysfunction
INTRODUCTION
Globally, both erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) are highly prevalent in men, and both conditions increase in prevalence with age [1–6]. Results from population–based surveys indicate that LUTS occur “at least sometimes” in up to 72% of middle–aged men [1, 2, 3]; while ED prevalence ranges from 2% in men younger than 40 years, to 86% in men 80 years and older [4]. Although methodological differences complicate comparisons, a recent survey of men aged 20–75 years in community and healthcare settings (without established urological disease) in the Russian Federation (N = 1225) detected ED symptoms in approximately 90% of respondents. Reasons for prevalent ED are speculative, but possible factors may include a population (Russian men) with a high rate of ED risk factors (e.g., cardiovascular disease) and ineffectiveness of traditional Russian ED treatments. The prevalence of urinary symptoms (as assessed by International Prostate Symptom Score [IPSS]) in the survey exceeded 60%, with moderate or severe symptoms in approximately 29% of the respondents [5]. The high prevalence of LUTS/BPH in the Russian sample may reflect, in part, subjective perception of the questions by respondents. Alternatively, a true higher prevalence of LUTS/BPH may exist given the relatively lower life expectancy of men in Russia compared to the West and earlier manifestation of different diseases.
Strong epidemiological evidence supports a correlation between LUTS and ED [7, 8]. The Cologne Male Survey of approximately 4,500 German men age 30– 80 years found that the prevalence of LUTS in men with ED was approximately 72% versus 38% in men with normal erections [9]. LUTS was an independent risk factor for ED [9]. In the Multinational Survey of the Aging Male (MSAM–7), a large study of over 12,000 respondents in six European countries and the United States, sexual disorders and their bothersomeness were strongly related to both age and severity of LUTS [10]. The presence and severity of LUTS were independent risk factors for sexual dysfunction. In Russia, epidemiological evidence also points to LUTS/BPH and ED comorbidity. A meta– analysis of survey data from a subset of men with “voiding dysfunction” (N = 767) found a “strong” correlation with ED symptoms in approximately 18% and “moderate” correlation in 10% of cases. Approximately two–thirds had a “weak” correlation between LUTS and ED symptoms [5]. The true correlation between LUTS and ED may be stronger than reported in this trial as patients in Russia may tend to mention only their primary complaint and omit concomitant symptoms as “less important” or “non– significant.”
Current practice patterns for BPH
In the presence of moderate or severe LUTS due to BPH, medical management has become the standard of care in patients not meeting criteria for surgical intervention [11, 12]. Nevertheless, there are variations among European countries concerning prescriptions related to BPH [13]. A retrospective analysis of European claims that the data (19 countries) found an increase in prescriptions for LUTS/ BPH, although prescription of BPH–related medications was “highly different” across Europe. Between 2004 and 2008, the number of BPH–related prescriptions increased by between 22% (France) and 145% (Hungary), with an increasing prescription gradient from northern to southern countries. Alpha1–adrenoceptor antagonists (alpha–blockers) were the most widely prescribed drugs, while use of 5–alpha–reductase inhibitors (5–ARIs) remained stable or slightly increased during the study period. Although not evidence– based, phytotherapy represented up to 40% of prescriptions and was country–specific.
Data on current treatment patterns for LUTS/BPH in Russia are sparse with wide region–specific variations. Available prescription data from five Russian cities in April 2007 indicated that alpha–blockers (40%) are an important component of medical management, with 5–ARIs (5%) or combination therapy (alpha–blockers + 5–ARIs) (10%) comprising a small proportion of prescriptions [14]. Phytotherapy is a major element of treatment, representing approximately 50% of prescriptions. At present, phosphodiesterase type 5 (PDE5) inhibitors or antimuscarinics comprise a small percentage of prescribed drugs for LUTS/BPH in Russia.
The 2013 Guidelines on the Management of Male LUTS (including Benign Prostatic Obstruction) published by the European Association of Urology (EAU) and guidelines compiled by the American Urological Association (AUA) recommend the use of several different pharmacotherapies for the treatment of LUTS, depending on the clinical situation [11, 12]. Alpha– blockers and 5–ARIs are considered the first–line medical treatment in men with moderate to severe lower urinary tract symptoms. The newest drug class, PDE5 inhibitors, are mentioned in the 2013 EAU guidelines. Per these guidelines, PDE5 inhibitors are limited to men who have LUTS and participate in clinical trials. Tadalafil gained first approval for LUTS/ BPH in October 2011 in the United States by the Food and Drug Administration (FDA) and was subsequently approved in the European Union, Russia, Canada, Israel, and several countries in Central and South America and Asia [15]. Russian regulatory authorities granted local approval for once–daily tadalafil for treatment of LUTS/BPH in January 2012.
Overlapping pathophysiologic processes
Several mechanisms have been proposed to explain the relationship between LUTS and ED (Figure 1), including pelvic reduction in nitric oxide synthase (NOS)/nitric oxide (NO) [16–20] and autonomic nervous system overactivity [16, 19, 20, 21]. Abnormal upregulation of the RhoA/Rho kinase (ROCK) pathway, which mediates smooth muscle contraction, might also contribute to the lack of smooth muscle relaxation in the prostate, urethra, bladder neck, and penis [17–20]. Upregulation of the ROCK pathway has been associated with pelvic ischemia and/ or atherosclerosis of blood vessels supplying pelvic organs [19], which is associated with ED and is a pathophysiologic risk factor for LUTS [16, 19, 20]. Altered androgen environment and inflammation represent additional pathophysiologic risk factors for LUTS and ED [16, 19].
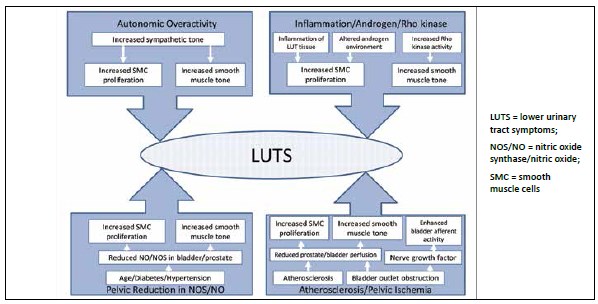
Figure 1. Overlapping pathophysiologic processes in LUTS and ED may include alteration in nitric oxide (NO) bioavailability, increased smooth muscle contractility, atherosclerosis, and changes in circulating sex hormone levels. Adapted from reference 19.
Because PDE5 is widely expressed in the bladder, prostate, and urethra, the smooth muscle and vasculature within these structures, it represents potential targets for PDE5 inhibitors [16]. PDE5 inhibitors have been shown to relax human smooth muscle samples from the prostate, bladder, and urethra by upregulating NO/cGMP activity [22, 23]. In addition, blocking PDE5 down regulates ROCK activity and has an antiproliferative effect in cultured human bladder cells [24]. PDE5 inhibitors were shown to increase prostate tissue oxygenation in a rat model of genitourinary ischemia/hypoxia [25] and increase cGMP release in ischemic LUT tissue in rabbits [26]. Clinically, a small study with 12 BPH patients awaiting surgery detected increased prostatic blood perfusion following tadalafil administration [27]. The possible effect of this phenomenon on subsequent blood loss during transurethral resection of the prostate has not been specifically evaluated. However, changes in organ perfusion represent normalization of the blood flow to support normal function; therefore, the potential for increased bleeding risk is not plausible. There have been no reports of increased bleeding during prostate surgery in men taking PDE5 inhibitors.
Afferent nerve activity represents another possible target of PDE5 inhibitors [16]. Animal models have shown reduced afferent nerve activity in the bladder following PDE5 inhibition [28, 29], and a specific action of tadalafil on chronic inflammation in the prostate was recently reported [30]. Thus, the mechanisms by which PDE5 inhibitors improve LUTS/ BPH are likely multifactorial: cGMP–mediated vascular relaxation, smooth muscle relaxation of the prostate and bladder, and decreased afferent nerve activity, which may complement vascular effects.
Clinical efficacy of tadalafil in LUTS/BPH
Clinical trials of PDE5 inhibitors in men with LUTS/ BPH have shown a reduction in symptoms as assessed by the IPSS questionnaire [31, 32] (Table 1). A large randomized study (N = 1058) of once–daily tadalafil versus placebo in patients with LUTS/ BPH showed statistically significant and clinically meaningful (≥3 point) improvement in total IPSS from baseline (tadalafil 2.5 mg, P = .015; 5–20 mg, all P < .001) over 12 weeks [33, 34]. Daily doses of 5, 10 and 20 mg tadalafil had very similar improvements in IPSS with “minimal” additional improvement in the 10– and 20–mg groups. The daily 2.5– mg dose did not consistently treat LUTS/BPH and is not recommended for this medical condition. In a post hoc analysis, changes in IPSS in men with and without ED (assessed by International Index of Erectile Function–Erectile Function [IIEF–EF]) were not significantly different (subgroup P = .352, subgroup–by–treatment P = .644) [35], which supports tadalafil’s improvement of LUTS independent of improved erectile function.
Table 1. Summary of clinical studies of once–daily tadalafil in LUTS/BPH. Adapted from references 33, 35–42, and 49
| Study | Population | N | Study Design/Treatment | Results | Comment |
| PBO–controlled Studies | |||||
| Roehrborn et al., 2008 [33] | LUTS/BPH | 1058 | Randomized, double–blind, PBO–controlled, dose– ranging (12 wks [EP]) Tadalafil 2.5, 5, 10 or 20 mg (QD) PBO |
Mean change IPSS (BL to EP)
|
|
| Broderick et al, 2010 [35] | LUTS/BPH with or without ED | 716 (ED) 340 (without ED) |
Post–hoc analysis: Randomized, double–blind, PBO–controlled, dose– ranging (12 wks [EP]) Tadalafil 2.5, 5, 10 or 20 mg (QD) PBO |
Mean change IPSS (BL to EP) With ED:
|
|
| Donatucci et al., 2011 [36] |
LUTS/BPH | 427 (who completed dose– ranging study) |
Open–label extension (52 wks [EP]) Tadalafil 5 mg (QD) (dose evaluated in open–label phase) PBO |
Mean change IPSS (Wk 0 to EP)
|
|
| Porst et al., 2011 [37] | LUTS/BPH (IPSS≥13, Qmax ≥4 to ≤15 mL/s) |
325 | Randomized, double–blind, PBO–controlled (12 wks [EP]) Tadalafil 5 mg (QD) PBO |
Mean change IPSS (BL to EP)
|
|
| Egerdie et al., 2012 [38] | LUTS/BPH with ED | 606 | Randomized, double–blind, PBO–controlled (12 wks [EP]) Tadalafil 2.5, 5 mg (QD) PBO |
Mean change IPSS (BL to EP) • Tadalafil 2.5 (–4.6, P=NS*) • Tadalafil 5 mg (–6.1, P<.001*) • PBO (–3.8) |
|
| Pooled Analyses | |||||
| Porst et al., 2013 [39] | LUTS/BPH with or without ED | 1500 (safety database) | Pooled data from 4 international, randomized, double–blind, PBO–controlled studies (12 wks [EP]) Tadalafil 5 mg (QD) PBO |
Mean change IPSS (BL to EP)
|
|
| Porst et al., 2013 [40] | LUTS/BPH with ED | 1026 | Pooled data from 4 international, randomized, PBO–controlled studies (12 wks [EP]); analyses restricted to men with ED Tadalafil 5 mg (QD) PBO |
Mean change IPSS (BL to EP)
|
|
| Brock et al., 2013 [41] | LUTS/BPH with or without ED | 1089 (balanced in baseline BPH severity) 751 (ED) 338 (without ED) |
Pooled data from 3 randomized, double–blind, PBO–controlled studies (12 wks [EP]) Tadalafil 5 mg (QD) PBO |
Mean change IPSS (BL to EP) With ED:
|
Improvement in LUTS/BPH similar in men with and without ED; P values for treatment–by– ED–status interactions were not significant for total IPSS (P=.73), IPSS voiding subscore (P=.69), IPSS storage subscore (P=.78), BPH impact index (P=.81), and IPSS QoL (P =.89) |
| Tadalafil and alpha–blocker comparator | |||||
| Oelke et al., 2012 [42] |
LUTS/BPH (IPSS≥13, Qmax ≥4 to ≤15 mL/s) |
511 | Randomized, double–blind, PBO–controlled (12 wks [EP]) Tadalafil 5 mg (QD) Tamsulosin 0.4 mg PBO |
Mean change IPSS (BL to EP)
|
|
| Combination treatment: Tadalafil with 5–alpha reductase inhibitor | |||||
| Casabé et al., 2014 [49] |
LUTS/BPH (IPSS ≥13, pros-tate vol-ume≥30 mL) |
695 | Randomized, dou-ble–blind, parallel (4, 12, 26 wks) Finasteride 5 mg/PBO Finasteride 5 mg/tadalafil 5 mg |
Mean change IPSS
|
|
Long–term efficacy of tadalafil was evaluated in an extension study (N = 427) of tadalafil 5 mg once–daily in men with LUTS/BPH [36]. Improvements during the placebo–controlled period or the first months of the extension were maintained during one year of therapy as assessed by total IPSS [36]. After 1 month of treatment in the extension phase, men who changed from placebo or 2.5 mg of tadalafil to the 5– mg dose had a statistically significant (P < .01) reduction in IPSS, while men who remained on tadalafil 5 mg or decreased their dose to 5 mg (from 10 or 20 mg) maintained the improvement. Data on tadalafil once–daily for LUTS/BPH efficacy with follow–up longer than 1 year are lacking.
The efficacy and safety of tadalafil in LUTS/BPH were investigated in a pivotal randomized, double–blind, placebo–controlled 12 week trial (N = 325) at a dose of 5 mg once–daily [37]. Tadalafil statistically significantly improved total IPSS from baseline to endpoint compared with placebo (P = .004), and the reduction in total IPSS was clinically meaningful [37]. Notably, total IPSS improved regardless of baseline ED severity. Another 12–week double–blind study evaluated the effect of tadalafil 2.5 mg or 5 mg once–daily on LUTS and ED in men with both conditions (N = 606) [38]. This study detected clinically meaningful and statistically significant total IPSS improvement with tadalafil 5 mg versus placebo at 2 weeks through endpoint P < .001). The 5–mg dose also significantly improved IPSS voiding (P < .001) and storage (P < .001) subscores versus placebo at 12 weeks.
Several integrated analyses of international studies have further evaluated tadalafil 5 mg once–daily versus placebo in LUTS/BPH in various subpopulations, including those with or without ED. A pooled analysis of four randomized, double–blind, placebo– controlled studies (N = 1500) of tadalafil 5 mg once–daily for LUTS/BPH confirmed statistically significant improvements from baseline in IPSS as well as BPH impact index and IPSS–QoL index (all P < .001) versus placebo [39]. Subgroup analyses found that IPSS improvements were significant regardless of baseline LUTS severity. A separate integrated analysis of data limited to sexually active men with ED (N = 1026) supported the observation that relief of LUTS with tadalafil treatment versus placebo (P < .001) is independent of improvement in ED [40]. There was no significant impact of baseline ED severity on IPSS response (interaction P value, 0.463), and improvement in IIEF–EF score was not significantly impacted by baseline LUTS/BPH severity (interaction P value, 0.926). Improvements in IPSS and IIEF–EF score during treatment were only weakly correlated (r = –0.229), which is consistent with no direct relationship between the magnitude of improvements in ED and LUTS/BPH. Some degree of correlation is expected when comorbid conditions are treated with a single agent.
Another pooled analysis of three global randomized, double–blind, placebo–controlled studies further supported the independent benefit of tadalafil in LUTS/ BPH and ED [41]. In these three similarly designed studies, a total of 1089 men (balanced in baseline BPH severity) with ED (n = 751) or without ED (n = 338) were randomly assigned to placebo or tadalafil 5 mg once–daily for 12 weeks. Regardless of the presence or absence of ED, treatment with tadalafil 5 mg resulted in significant improvement in total IPSS mean change from baseline compared with placebo, and significant improvements in IPSS subscores (voiding and storage) and quality of life. Notably, improvement in LUTS/BPH was similar in men without and with ED, as the P values for treatment–by–ED–status interactions was not significant for total IPSS (P = .73).
Tadalafil with or without an alpha–blocker
Given that alpha–blockers are often first–line treatments for LUTS/BPH, tamsulosin 0.4 mg once–daily was included as the active control in a double–blind placebo–controlled study (N = 511) of tadalafil 5 mg for 12 weeks [42]. Although not designed for statistical testing of superiority, this study found that the change from baseline to week 12 in total IPSS was statistically significant for both tadalafil and tamsulosin versus placebo (P = .001 and P = .023, respectively). The magnitude of improvement in total IPSS at the 12–week endpoint with tadalafil was comparable to that with tamsulosin and consistent with other reports [33, 37, 38]. As measured by IIEF–EF, tadalafil significantly improved erectile function (P < .001) compared with placebo in sexually active men with ED (approximately 60% of subjects), while tamsulosin did not (P = .699) [42, 43]. Overall, evidence from this study indicates that tadalafil 5 mg or tamsulosin 0.4 mg once–daily results in similar improvements in LUTS/BPH symptoms at 12 weeks. Well–controlled clinical trials that thoroughly evaluate the potential action of combination therapy with tadalafil and an alpha–blocker are lacking. A preliminary report of alfuzosin 10 mg once–daily, tadalafil 20 mg on alternative days, or a combination of both in men with LUTS/BPH indicated that co–therapy improved symptom and uroflowmetry measures [44]. A small placebo–controlled study (N = 40) of tamsulosin 0.4 mg/tadalafil 5 mg versus tamsulosin 0.4 mg/ placebo once–daily in LUTS/BPH detected a significant decrease in total IPSS (P = .01) from baseline to week 4 in the tamsulosin/tadalafil group [45]. A small crossover study (N = 30) of tamsulosin 0.4 mg/ tadalafil 20 mg or tamsulosin 0.4 mg/placebo once– daily in LUTS/BPH showed significant improvements in IPSS with both treatments [46]. Evidence from these non–registration studies suggests an additive effect with combination therapy. However, the co–therapy database is limited and further research is needed. The manufacturer of tadalafil has not conducted a well controlled head–to–head or combination study with alpha–blockers other than for safety reasons. At present, prescribing instructions do not recommend tadalafil in combination with alpha– blockers in LUTS/BPH because efficacy has not been adequately studied and because of the risk of blood pressure lowering [47].
Tadalafil with 5–alpha reductase inhibitor
Given their distinct mechanisms of action, addition of a PDE5 inhibitor to a 5–ARI may offer earlier symptomatic relief in LUTS. Additionally, the use of tadalafil may reduce the sexual side effects (including ED) observed with 5–ARIs [48]. To investigate this combination, 5–ARI–naïve men with LUTS (N = 695) secondary to BPH were randomized to finasteride 5 mg/placebo or finasteride 5 mg/tadalafil 5 mg following placebo run–in in a double–blind, parallel group study [49]. Tadalafil 5 mg once–daily, co–administered with finasteride 5 mg for 12 weeks resulted in total IPSS improvement that was significantly better than finasteride/placebo (P = .001) [49]. Significantly greater LUTS improvements were also observed with finasteride/tadalafil at both 4 weeks (P < .001) and 26 weeks (P = .022) post–baseline. The data also indicated that quality of life was enhanced with finasteride/tadalafil therapy. The IPSS–QoL index was numerically improved with finasteride/ tadalafil compared with finasteride/placebo at all three postbaseline assessments and reached statistical significance at week 4 (P < .001) [49]. Improvement in QoL approached significance at 12 weeks (P = .051). Among sexually active patients who had ED at baseline, finasteride/tadalafil led to significant improvements in IIEF–EF scores at all three postbaseline time points compared with finasteride/placebo (all P <.001).
The frequency of mild to moderate adverse events (AEs) with tadalafil in LUTS/BPH was similar to the frequency with placebo in trials encompassing a broad demographic spectrum and disease severity [33, 37, 38, 50]. No clear dose relationship to safety was evident in dose–finding studies [33, 50] or in a urodynamic safety study in which a high dose of tadalafil (20 mg) was administered for 12 weeks [51]. In studies with long–term extensions, adverse events with tadalafil 5 mg were similar to those reported during the double–blind phase [36, 50] (Table 2). Together, these findings suggest that the 5–mg daily dose of tadalafil for treatment of LUTS/BPH possesses the best benefit–risk profile.
The safety and tolerability of co–administered PDE5 inhibitors and 5–ARIs or alpha–blockers remain to be fully explored. In the single study of finasteride/tadalafil, the overall safety profile did not deviate from what had previously been established for tadalafil and finasteride monotherapy. Most treatment–emergent adverse events (TEAEs) were mild– to– moderate in severity, and the incidence of serious AEs and discontinuations due to AEs were low and not significantly different between treatment groups [49]. Patients receiving the finasteride/tadalafil combination had fewer events of reduced libido or ED versus finasteride/ placebo.
Since alpha–blockers have vasodilatory effects and PDE5 inhibitors are mild vasodilators, an additive decrease in blood pressure leading to symptomatic hypotension is a potential side effect. However, results of a 12–week randomized, double–blind study (N = 318) found similar changes in hemodynamic signs and symptoms in men receiving tadalafil 5 mg or placebo in combination with stable alpha– blocker therapy [52]. The proportion of patients who reported treatment–emergent dizziness was not significantly different (tadalafil/alpha–blocker 7.0%; placebo/alpha–blocker 5.7%; P = .403). In a secondary analysis of TEAEs possibly related to hypotension, no differences were observed between treatment groups with respect to patients meeting the criteria for a positive orthostatic test (P = 1.00). However, when stratified by alpha–blocker type, a greater proportion of tadalafil patients taking a non–uroselective alpha–blocker (such as doxazosin) reported one or more positive orthostatic tests compared to patients taking placebo (Figure 2). Nevertheless, no apparent dose relationship was observed when examining those taking doxazosin ≤4 mg versus >4 mg, and there was no temporal association between symptoms and changes in vital signs. More research is needed to identify the potential for hypotensive events with tadalafil and alpha–blocker co–therapy, particularly if a non–uroselective agent is selected.
Implications for clinical treatment of LUTS/BPH in Russia
Clinical practice patterns for the treatment of LUTS/ BPH in Russia reflect the use of conventional medical therapies identified in the EAU and other guidelines as well as therapies distinct to the Russian setting. Although not evidence–based, phytotherapy and food supplements are frequently recommended forms of treatment by doctors and pharmacists in Russia. The reason for this phenomenon is complex and includes aggressive advertising by manufacturers and insufficient financial government support for selected low–income patient categories. Given the current burden of LUTS secondary to BPH in middle–age and elderly men in Russia, unmet needs exist that are not being addressed by current treatment practices. As the only PDE5 inhibitor currently approved for the treatment of LUTS due to BPH, tadalafil represents an option either as monotherapy or combined with conventional agents, primarily in men that suffer both LUTS/BPH and ED. There is mounting evidence that the clinical benefits of tadalafil in LUTS and erectile function are independent.
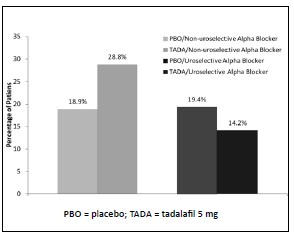
Figure 2. One or more positive orthostatic tests in men with LUTS/BPH receiving daily alpha–blockers and tadalafil 5 mg. Adapted from reference 52.
Table 2. Most common (≥2%) treatment–emergent adverse events in the open–label extension phases of multinational and
Japanese dose–finding studies. Adapted from references 36 and 50
| Multinational dose–finding study (open–label phase) Tadalafil 5 mg* (N=427) |
Japanese dose–finding study (open–label phase) Tadalafil 5 mg* (N=394) |
|||
| One or more TEAE, n (%) | 246 (57.6) | 232 (58.9) | ||
| Dyspepsia | 17 (4.0) | Nasopharyngitis | 42 (10.7) | |
| GERD | 17 (4.0) | Diarrhea | 24 (6.1) | |
| Back pain | 16 (3.7) | Back pain | 17 (4.3) | |
| Headache | 13 (3.0) | Headache | 12 (3.0) | |
| Sinusitis | 12 (2.8) | Dyspepsia | 10 (2.5) | |
| Hypertension | 11 (2.6) | Eczema | 9 (2.3) | |
| Cough | 9 (2.1) | Insomnia | 8 (2.0) | |
| Reflux esophagitis | 8 (2.0) | |||
| Discontinuation for AE, n (%) | 22 (5.2) | 36 (9.1) | ||
| One or more SAE, n (%) | 20 (4.7) | 11 (2.8) | ||
AE – adverse events; GERD – gastroesophageal reflux disease; SAE – serious adverse events;
TEAE – treatment emergent adverse event
*Continued or switched to 5 mg dose
Tadalafil does not represent direct competition with conventional medical therapies for LUTS. Further studies on the use of combination therapy with tadalafil and an alpha–blocker or 5–ARI are required. If these combinations are confirmed as a viable option based on well–designed and powered clinical studies, once–daily tadalafil dosing, which coincides with regimens of commonly used agents, can facilitate combination therapy in patients who are candidates for more aggressive treatment and/or suffer from sexual side effects of conventional medical therapy. In summary, tadalafil results in symptom improvements across a range of male patients with LUTS/BPH and represents a new treatment option in Russian men who are candidates for medical therapy.
References
- Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009; 104: 352–360.
- Coyne KS, Sexton CC, Bell JA, Thompson CL, Dmochowski R, Bavendam T, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB–POLL. Neurourol Urodyn. 2013; 32: 230–237.
- Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol. 2012; 188: 496–501.
- Prins J, Blanker MH, Bohnen AM, Thomas S, Bosch JL. Prevalence of erectile dysfunction: a systematic review of population–based studies. Int J Impot Res. 2002; 14: 422–432.
- Pushkar DY, Kamalov AA, Al–Shukri SH, Erkovich AA, Kogan MI, Pavlov VN, et al. [An epidemiological study of the prevalence of erectile dysfunction in the Russian Federation]. Russ Med J. 2012; 112.
- Kubin M, Wagner G, Fugl–Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res. 2003; 15: 63–71.
- Seftel AD, de la Rosette J, Birt J, Porter V, Zarotsky V, Viktrup L. Coexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological data. Int J Clin Pract. 2013; 67: 32–45.
- Ponholzer A, Temml C, Obermayr R, Madersbacher S. Association between lower urinary tract symptoms and erectile dysfunction. Urology. 2004; 64: 772–776.
- Braun MH, Sommer F, Haupt G, Mathers MJ, Reifenrath B, Engelmann UH. Lower urinary tract symptoms and erectile dysfunction: comorbidity or typical 'aging male' symptoms? Results of the 'Cologne Male Survey'. Eur Urol. 2003; 44: 588–594.
- Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM–7). Eur Urol. 2003; 44: 637–649.
- Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. M. Guidelines on the management of male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO). European Association of Urology. 2013. Available from http://www.uroweb.org/gls/ pdf/13_Male_LUTS_LR.pdf. Accessed July 2, 2013.
- McVary KT, Roehrborn CG, Avins AL, Barry J, Brsuskewitz RC, Donnel RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011; 185: 1793–1803.
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. Eur Urol. 2010; 58: 450–456.
- Synovate Comcon. PrIndex “Prescription monitoring of drugs” 5 cities. April 2007. http://eng.synovate.ru/?trID=339
- PRNewswire (Oct. 30, 2012). European Commission approves Cialis® (tadalafil) tablets for the treatment of the signs and symptoms of benign prostatic hyperplasia. Available from http://newsroom.lilly.com/ releasedetail.cfm?releaseid=717071. Accessed April 4, 2013.
- Giuliano F, Ückert S, Maggi M, Birder L, Kissel J, Viktrup L. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013; 63: 506–516.
- McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005; 47: 838–845.
- Gacci M, Eardley I, Giuliano F, Hatzichristou D, Kaplan SA, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011; 60: 809–825.
- Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, Roehrborn CG, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011; 30: 292–301.
- Kim SD, Park JW. Role of phosphodiesterase type 5 inhibitor on benign prostatic hyperplasia/lower urinary tract symptoms. Korean J Androl. 2011; 29: 91–100.
- Yassin A, Saad F, Hoesl CE, Traish AM, Hammadeh M, Shabsigh R. Alpha–adrenoceptors are a common denominator in the pathophysiology of erectile function and BPH/LUTS––implications for clinical practice. Andrologia. 2006; 38: 1–12.
- Uckert S, Sormes M, Kedia G, et al. Effects of phosphodiesterase inhibitors on tension induced by norepinephrine and accumulation of cyclic nucleotides in isolated human prostatic tissue. Urology. 2008; 71: 526–530.
- Kedia GT, Sonnenberg JE, Kuczyk MA, Uckert S. In vitro functional responses of isolated human urethral tissue to phosphodiesterase (PDE) inhibitors (Abstract 931). Eur Urol Suppl. 2011; 10: 291–292.
- Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, et al. Characterization and functional role of androgen–dependent PDE5 activity in the bladder. Endocrinology. 2007; 148: 1019–1029.
- Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, Gacci M, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011; 8: 2746–2760.
- Azadzoi KM, Babayan RK, Kozlowski R, Siroky MB. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003; 170: 659–663.
- Bertolotto M, Trincia E, Zappetti R, Bernich R, Savoca G, Cova MA. Effect of tadalafil on prostate haemodynamics: preliminary evaluation with contrast–enhanced US. Radiol Med. 2009; 114: 1106–1114.
- Behr–Roussel D, Oger S, Caisey S, Sandner P, Bernabé J, Alexandre L, Giuliano F. Vardenafil decreases bladder afferent nerve activity in unanesthetized, decerebrate, spinal cord–injured rats. Eur Urol. 2011; 59: 272–279.
- Minagawa T, Aizawa N, Igawa Y, Wyndaele JJ. Inhibitory effects of phosphodiesterase 5 inhibitor, tadalafil, on mechanosensitive bladder afferent nerve activities of the rat, and on acrolein–induced hyperactivity of these nerves. BJU Int. 2012; 110: E259–266.
- Gacci M, Salvi M, Sebastianelli A, Vignozzi L, Corona G, McVary KT, et al. The use of a single daily dose of tadalafil to treat signs and symptoms of benign prostatic hyperplasia and erectile dysfunction. Res Rep Urol. 2013; 5: 99–111.
- Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, Kaplan SA, et al. A systematic review and meta–analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α–blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2012; 61: 994–1003.
- Martínez–Salamanca JI, Carballido J, Giuliano F, Gratzke C, Rosen R, et al. Phosphodiesterase type 5 inhibitors in the management of non–neurogenic male lower urinary tract symptoms: critical analysis of current evidence. Eur Urol. 2011; 60: 527–535.
- Roehrborn CG, McVary KT, Elion–Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008; 180: 1228–1224.
- American Urological Association (AUA). Guideline on the management of benign prostatic hyperplasia (BPH). Available from http://www. auanet.org/content/guidelines– and–quality–care/clinical–guidelines/ main– reports/bph–management/chap_1_GuidelineManagementof (BPH).pdf. Updated 2010. Accessed July 20, 2011.
- Broderick GA, Brock GB, Roehrborn CG, Watts SD, Elion–Mboussa A, Viktrup L. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia in men with or without erectile dysfunction. Urology. 2010; 75: 1452–1458.
- Donatucci CF, Brock GB, Goldfischer ER, Pommerville PJ, Elion–Mboussa A, Kissel JD, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a 1 year open–label extension study. BJU Int. 2011; 107: 1110–1116.
- Porst H, Kim ED, Casabй AR, Mirone V, Secrest RJ, Xu L, Sundin DP, Viktrup L; LVHJ study team. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of a multinational randomized, double–blind, placebo–controlled trial. Eur Urol. 2011; 60: 1105–1113.
- Egerdie RB, Auerbach S, Roehrborn, Costa P, Garza MS, Esler AL, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo–controlled, double–blind study. J Sex Med. 2012; 9: 271–281.
- Porst H, Oelke M, Goldfischer ER, Cox D, Watts S, Dey D, Viktrup L. Efficacy and safety of tadalafil 5 mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analyses of pooled data from 4 multinational, randomized, placebo–controlled clinical studies. Urology. 2013; 82: 667–673.
- Porst H, Roehrborn CG, Secrest RJ, Esler A, Viktrup L. Effects of tadalafil on lower urinary tract symptoms secondary to benign prostatic hyperplasia and on erectile dysfunction in sexually active men with both conditions: analyses of pooled data from four randomized, placebo–controlled tadalafil clinical studies. J Sex Med. 2013; 10: 2044–2052.
- Brock G, Broderick G, Roehrborn CG, Xu L, Wong D, Viktrup L. Tadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunction. BJU Int. 2013; 112: 990–997.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo–controlled clinical trial. Eur Urol. 2012; 61: 917–925.
- Giuliano F, Oelke M, Jungwirth A, Hatzimouratidis K, Watts S, Cox D, Viktrup L. Tadalafil once daily improves ejaculatory function, erectile function, and sexual satisfaction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia and erectile dysfunction: results from a randomized, placebo– and tamsulosin–controlled, 12–week double–blind study. J Sex Med. 2013; 10: 857–865.
- Liguori G, Trombetta C, De Giorgi G, Pomara G, Maio G, Vecchio D, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med. 2009; 6: 544–552.
- Regadas RP, Reges R, Cerqueira JB, Sucupira DG, Josino IR, Nogueira EA, et al. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo–controlled clinical trial. Int Urol Nephrol. 2013; 45: 39–43.
- Bechara A, Romano S, Casabé A, Haime S, Dedola P, Hernández C, Rey H. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 2008; 5: 2170–2178.
- CIALIS® (tadalafil) Prescribing Information. Eli Lilly and Company. Revised October 2013. http://pi.lilly.com/us/cialis–pi.pdf
- Miller MS. Role of phosphodiesterase type 5 inhibitors for lower urinary tract symptoms. Ann Pharmacother. 2013; 47: 278–283.
- Casabé A, Roehrborn CG, Da Pozzo LF, Zepeda S, Jonathan Henderson RJ, Sorsaburu S, et al. Efficacy and safety of the co–administration of tadalafil once daily with finasteride for 6 months: a randomized, double–blind, placebo–controlled study in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol. 2014; 191: 727–733.
- Takeda M, Nishizawa O, Imaoka T, Morisaki Y, Viktrup L. LVIA: Tadalafil for the treatment of lower urinary tract symptoms in Japanese men with benign prostatic hyperplasia; results from a 12–week placebo–controlled dose–finding study and a 42–week open–label extension. LUTS. 2012; 3: 110–119.
- Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12–week clinical trial. J Urol. 2010; 183: 1092–1097.
- Goldfischer E, Kowalczyk JJ, Clark WR, Brady E, Shane MA, Dgetluck N, Klise SR. Hemodynamic effects of once–daily tadalafil in men with signs and symptoms of benign prostatic hyperplasia on concomitant α1– adrenergic antagonist therapy: results of a multicenter randomized, double–blind, placebo–controlled trial. Urology. 2012; 79: 875–882.






